AP Physics: Board Meeting
Students whiteboarded their results from a Pivot Interactives activity on Coulomb’s Law. There was some debate over whether inverse or inverse-square was the right linearization; I usually don’t have students sketch their points on their whiteboards, but I think that would have been helpful today. Students did a nice job connecting their results to Newton’s Laws and their knowledge from chemistry.

This group ran out of space for their linearization, but I found their set of graphs very satisfying.
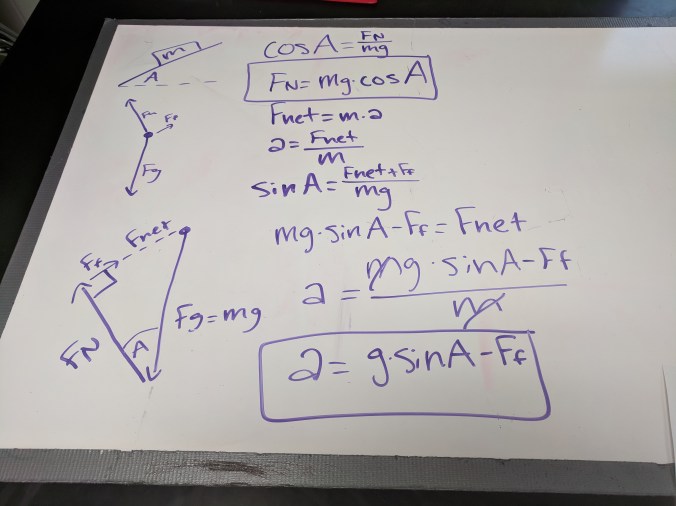
Physics: Dissipated Energy
We continued prep for determining which interaction causes a bouncy ball to dissipate energy (my article about this activity was published in the January issue of The Science Teacher) by whiteboarding key points of yesterday’s work. Today really seemed to help a lot of students see the connections between the energy bar charts, free-body diagrams, and velocity vs. time graphs, which is exactly what I was going for.

Chemistry Essentials: Mistakes Game
We used the mistakes game to go over yesterday’s problems. There was some great discussion, but it was very tough to keep students from breaking into side conversations. Next time, I should spend a little more time making sure behavior expectations are explicit as possible and helping students see the value in those expectations. There were also some students who were extremely engaged and clearly developed a lot of confidence in sketching Borh models today, which was awesome.