Today was students’ last day before a 3-day weekend and our homecoming pepfest, so classes were short and students were more energetic than usual.
AP Physics: Board Meeting
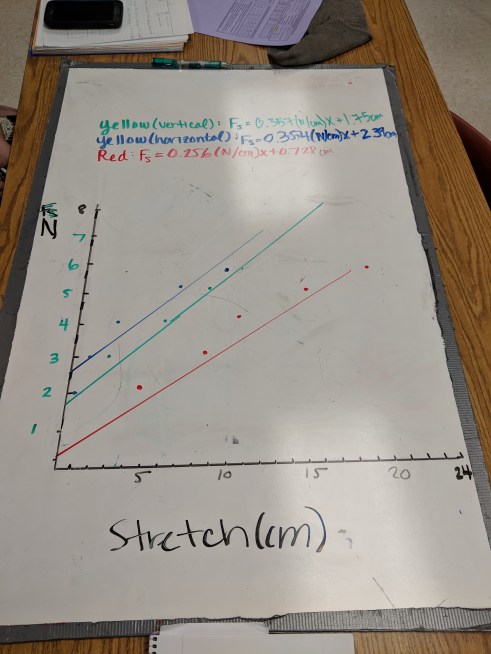
We had a board meeting for the spring force lab. Students initiated some good discussion about the intercepts in both sections, but I had to do a little coaxing to get them thinking about the values of the slopes. One of the challenges was a lot of groups hadn’t distinguished between mass and force of gravity, which tells me I should have done a little more pre-lab discussion, especially since that distinction was just introduced earlier this week.
Physics: Annotating Graphs
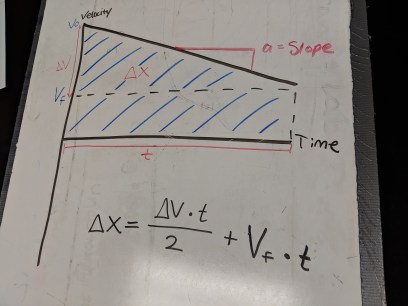
Students whiteboarded yesterday’s problems for a short gallery walk before trying some calculations. I think this is the first year where I didn’t have any students opt to use the formula for the area of a trapezoid on any of their graphs; it just felt more natural to most of my students to split the graph into a triangle and rectangle (which is what I usually do). When students started working with numbers, I had a lot of students independently start talking about specific times and velocities as coordinate pairs, which I haven’t seen students do before and was pretty great.
Chemistry Essentials: Gas Laws
Students sketched their graphs from the past few days for a simplified board meeting. One of the things I really appreciate about this group is I have some students who are really willing to speak up when they are confused about something; one of my students was struggling to see how the graphs fit with the qualitative relationships we found earlier this week and didn’t hesitate to say so, which lead to some valuable discussion about how to read a graph.
























