AP Physics 1: Board Meeting
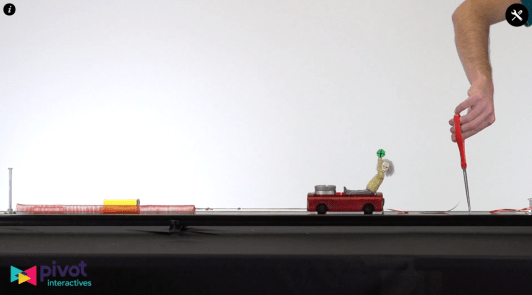
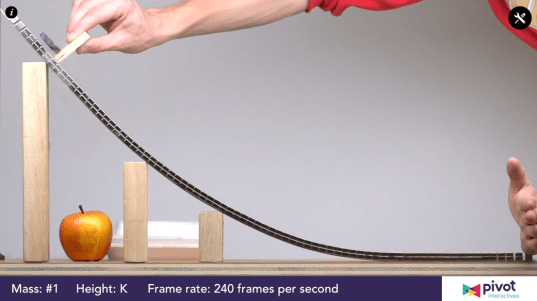
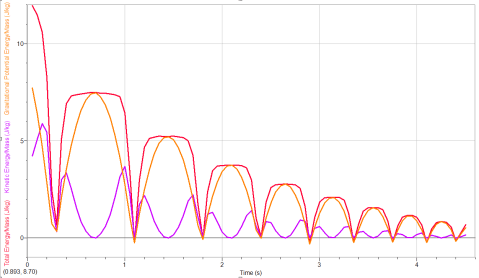
Students whiteboarded their results for the elastic potential energy lab we’d done earlier this week.
Physics: Representations Jeopardy
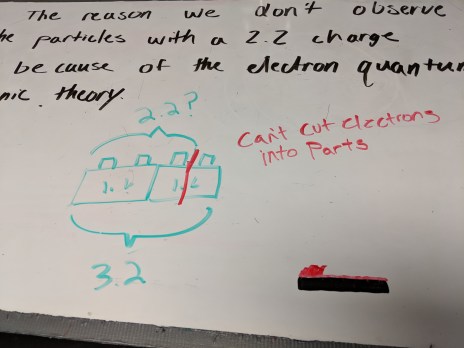
In both my sections, we started with mistakes whiteboarding for yesterday’s problems. My 6th hour is about 1/3 the size of my other section, so they got through the mistakes whiteboarding very quickly. I tried what I called Representations Jeopardy: each group came up with a scenario, and whiteobarded a set of representations, minus the sketches and any labels that would identify what the objects involved are. Then, groups traded whiteboards and had to come up with a scenario that matched the whiteboard they received. Students said they really liked that they had to think differently in order to work backwards from the diagrams.

From mistakes whiteboarding
Chemistry Essentials: Density of a Solid
Students worked on finding the density of some metal dowels. I realized belatedly this is the first lab we’ve done where they didn’t need a container when measuring the mass, so it was actually a tricky leap for them to not tare something out on the balance.