AP Physics: Multiple Choice
After a quiz on projectile motion graphs, we spent some time using Plickers to practice multiple choice on energy and projectiles. One of my classes pretty openly started guessing, rather than thinking about the problems, so I think we may be doing multiple choice a little too regularly. I may start either alternating each week between relevant multiple choice and free response or just use quiz days for explicit AP practice less often.
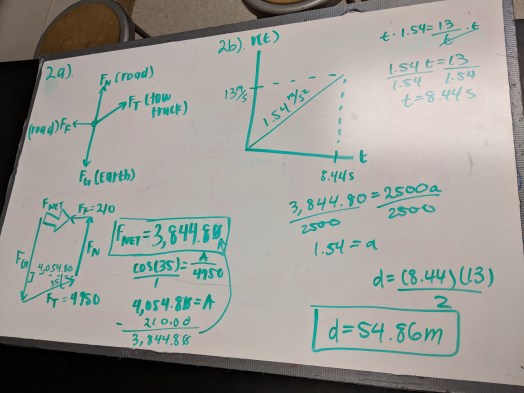
Physics: Forces
Before today’s quiz, students whiteboarded their diagrams for the problems earlier this week. Pretty consistently at this point, the students who take the time to get their diagrams right do fine on the calculations, which is not surprising. Getting students to put units in their work is still a challenge, but I saw a lot more confidence from my students today than I have in a while.
Chemistry Essentials: Gas Laws
Before today’s quiz, we did a quick debrief of yesterday’s lab on the gas laws. Since the ice water didn’t work well yesterday, I tried putting them outside (the air temperature was -10 degrees F today!), but still didn’t see much change, so I think the syringes I have just don’t seal well enough. We’ll finish the post-lab discussion on Monday.