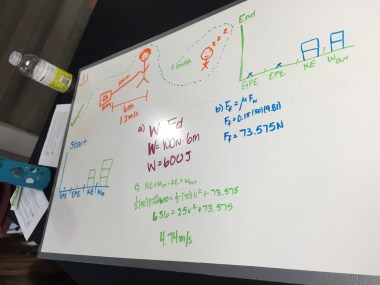
Physics: Projectile Launchers
Students tested the final versions of their projectile launchers today. I picked random target distances in three different ranges, and groups had to pick one target to try and hit. I really enjoyed seeing the creativity in the materials students used. On group built a basic launcher out of K’Nex and decided to take a slushie break as they tried to come up with something to hold the ping pong ball, only to realize the lids from their slushies were the perfect size and shape. They also decided an adjustable height would make it easier to hit the target and came up with attaching the launcher to a music stand.
While students applied a lot of good data analysis to the project, the connections to energy and projectile motion weren’t as strong as I’d like, which I think has a lot to do with the way I broke up the project. I tried to squeeze energy into the two weeks between the start of tri 2 and winter break, and the project ended up bleeding into electrostatics. Next year, I may try starting the project with projectile motion, then working the redesign into the energy unit. There will be some administrative challenges, since the project will be split across two terms and a lot of students switch class periods, but I think it will pay off with students seeing a stronger connection to the physics content.

Music stand, K’Nex, and a slushie lid
Chemistry: Return to Stoich
The next unit covers percent yield and limiting reagents, but scores were low enough on the assessment I gave before winter break that I want to revisit stoichiometry before we try any percent yield problems. Today, students started a lab to predict masses for an imaginary reaction using nuts, bolts, and washers in place of actual atoms. I wanted to give them something they could manipulate and measure very directly to get some conceptual understanding of stoich before we try any more problems. I wrote the lab with the intention of helping students work piece by piece through the process and questions frequently rely on information from a previous answer. A few groups, I think in an effort to be collaborative, tried to divide and conquer, and the students working on the later portions of the lab found they were stuck. Before we finish the lab next week, I’m planning to have a conversation with them about the shortfalls of the divide and conquer strategy and to come up with some more effective collaboration strategies.